
Three things to take away
What is the outlook for the global life sciences sector – and how is technology impacting growth and development
What are the key areas of advancement where AI integrates with the life sciences sector
What are the emerging risks and what is the impact on adjacent industries, including insurance
In the ever-evolving landscape of the life sciences world, the integration of AI and other cutting-edge technologies promises to reshape the healthcare industry's approach to patient centricity. From streamlining clinical trials to significantly enhancing drug design and device development, the disruption potential related to current innovation trends is vast and promising.
But how will it impact insurance? What are the likely pivotal market implications, thinking specifically about emerging risks and potential opportunities?
What is the outlook for the global life sciences sector – and how is technology impacting growth and development
What are the key areas of advancement where AI integrates with the life sciences sector
What are the emerging risks and what is the impact on adjacent industries, including insurance
In the ever-evolving landscape of the life sciences world, the integration of AI and other cutting-edge technologies promises to reshape the healthcare industry's approach to patient centricity. From streamlining clinical trials to significantly enhancing drug design and device development, the disruption potential related to current innovation trends is vast and promising.
But how will it impact insurance? What are the likely pivotal market implications, thinking specifically about emerging risks and potential opportunities?
What is the life sciences industry?
The life sciences industry represents one of the dominant economic sectors in the world. ‘Health life sciences’ refers to the application of biology and technology to health improvement in humans and animals. Typical products would include biotechnology, pharmaceuticals, medical devices class 1, 2 and 3, healthcare information technology and nutraceuticals.
In addition, it also covers professional services such as clinical manufacturing organizations, distributors, diagnostics, and clinical research organizations.
The life sciences industry represents one of the dominant economic sectors in the world. ‘Health life sciences’ refers to the application of biology and technology to health improvement in humans and animals. Typical products would include biotechnology, pharmaceuticals, medical devices class 1, 2 and 3, healthcare information technology and nutraceuticals.
In addition, it also covers professional services such as clinical manufacturing organizations, distributors, diagnostics, and clinical research organizations.
Promising trend
The integration of artificial intelligence (AI) and other cutting-edge technologies promises to reshape the healthcare industry in many ways, particularly with regards to its approach to patient centricity. Factors driving the use of AI in life sciences include significant growth in healthcare data availability, major advances in AI algorithms, and the political and moral imperative to address unmet medical needs more efficiently and effectively around the world.
Healthcare data availability has increased substantially over the past decades. This is a result of the continuous evolution of a myriad of source collection mechanisms, including electronic health records, genomic sequencing data, medical imaging studies and wearable sensors. This has allowed researchers and practitioners to improve their understanding of diseases, potential treatments, and patient responses. Processing an ever-growing set of data in impactful ways has been a challenge. However, innovative solutions powered by AI are now promising to provide a solution to that.
Machine learning algorithms, which enable computers to learn from data and make predictions or decisions without explicit programming, have made remarkable strides in recent years – as widely evidenced by recently released generative AI tools such as ChatGPT. Today’s AI algorithms can analyze vast datasets with unprecedented speed and accuracy, uncovering patterns, correlations and insights that are not easily accessible or identifiable for humans. These advances have unlocked new possibilities across various domains within the life sciences realm, from drug discovery and clinical trials to personalized medicine and medical device customization.
AI algorithms can analyze vast datasets with unprecedented speed and accuracy
Fig 1: Likely benefits of AI integration in the life science world
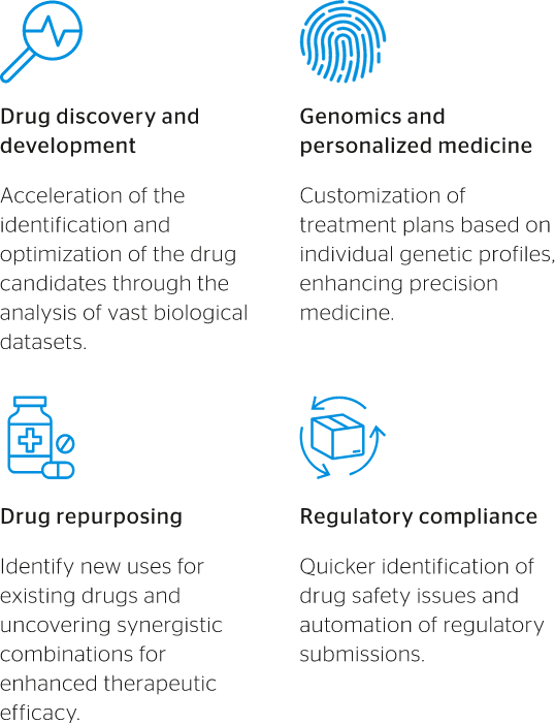
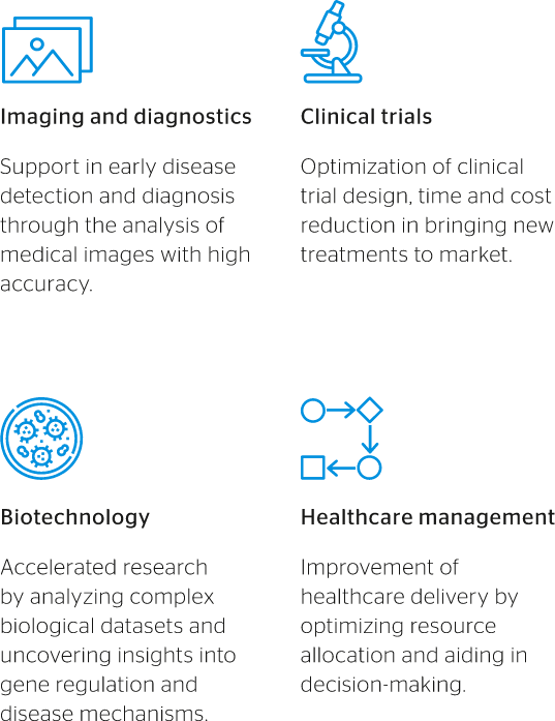
The integration of artificial intelligence (AI) and other cutting-edge technologies promises to reshape the healthcare industry in many ways, particularly with regards to its approach to patient centricity. Factors driving the use of AI in life sciences include significant growth in healthcare data availability, major advances in AI algorithms, and the political and moral imperative to address unmet medical needs more efficiently and effectively around the world.
Healthcare data availability has increased substantially over the past decades. This is a result of the continuous evolution of a myriad of source collection mechanisms, including electronic health records, genomic sequencing data, medical imaging studies and wearable sensors. This has allowed researchers and practitioners to improve their understanding of diseases, potential treatments, and patient responses. Processing an ever-growing set of data in impactful ways has been a challenge. However, innovative solutions powered by AI are now promising to provide a solution to that.
Machine learning algorithms, which enable computers to learn from data and make predictions or decisions without explicit programming, have made remarkable strides in recent years – as widely evidenced by recently released generative AI tools such as ChatGPT. Today’s AI algorithms can analyze vast datasets with unprecedented speed and accuracy, uncovering patterns, correlations and insights that are not easily accessible or identifiable for humans. These advances have unlocked new possibilities across various domains within the life sciences realm, from drug discovery and clinical trials to personalized medicine and medical device customization.
AI algorithms can analyze vast datasets with unprecedented speed and accuracy
Fig 1: Likely benefits of AI integration in the life science world


Key areas of advancement
Drug discovery
AI has the potential to significantly reduce the time and cost required to bring new drugs to market, while also increasing the likelihood of success.
Historically, the journey from drug discovery to market approval has been characterized by lengthy timelines, exorbitant costs, and elevated failure rates. According to different sources, the cost of bringing a new drug to market often exceeds a billion dollars, with development timelines spanning 10-15 years on average.
However, AI integration is set to change that. AI-driven drug discovery platforms leverage sophisticated algorithms to analyze vast libraries of molecular structures, predict the biological activity of potential drug candidates, and optimize their chemical properties for efficacy and safety. These platforms enable researchers to identify promising drug candidates more efficiently, prioritize lead compounds for further evaluation, and expedite pre-clinical and clinical development stages.

Drug discovery
AI has the potential to significantly reduce the time and cost required to bring new drugs to market, while also increasing the likelihood of success.
Historically, the journey from drug discovery to market approval has been characterized by lengthy timelines, exorbitant costs, and elevated failure rates. According to different sources, the cost of bringing a new drug to market often exceeds a billion dollars, with development timelines spanning 10-15 years on average.
However, AI integration is set to change that. AI-driven drug discovery platforms leverage sophisticated algorithms to analyze vast libraries of molecular structures, predict the biological activity of potential drug candidates, and optimize their chemical properties for efficacy and safety. These platforms enable researchers to identify promising drug candidates more efficiently, prioritize lead compounds for further evaluation, and expedite pre-clinical and clinical development stages.

Clinical trials
AI also holds the potential to revolutionize clinical trials, which remain marked by challenges and bottlenecks such as recruitment delays, inefficient protocols, and high costs. According to a March 2024 article published in the magazine Nature, over the past six decades the number of drugs approved in the US per billion dollars spent in research and development has halved every nine years. Similar scenarios have been seen in other markets as well, such as Europe, where a combination of red tape and operational complexities has sustained elevated costs for clinical trials and drug development more broadly.
By leveraging AI-driven analytics, researchers can enhance patient recruitment strategies, identify suitable candidates more efficiently, and optimize trial protocols to ensure faster, more reliable results. AI-based predictive modelling will also help researchers to better anticipate patient dropout rates, optimize trial endpoints, and identify biomarkers of treatment response, facilitating more informed decision making throughout the trial process. Finally, AI-driven virtual trials, leveraging real-world data and digital endpoints, have the potential to streamline trial logistics, reduce costs, and enhance participant engagement.
System.AggregateException: One or more errors occurred. (Value cannot be null. (Parameter 'input')) ---> System.ArgumentNullException: Value cannot be null. (Parameter 'input') at System.Text.RegularExpressions.ThrowHelper.ThrowArgumentNullException(ExceptionArgument arg) at System.Text.RegularExpressions.Regex.Matches(String input) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.FindLocalLinkIds(String text)+MoveNext() at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.Convert(Object source, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) at QBEEurope.Core.ValueConverters.GlobalVariablesValueConverter`1.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Core\ValueConverters\GlobalVariablesValueConverter.cs:line 99 at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.<.ctor>b__7_1() at System.Lazy`1.ViaFactory(LazyThreadSafetyMode mode) at System.Lazy`1.ExecutionAndPublication(LazyHelper executionAndPublication, Boolean useDefaultConstructor) at System.Lazy`1.CreateValue() at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.GetValue(String culture, String segment) at Umbraco.Extensions.PublishedPropertyExtension.Value[T](IPublishedProperty property, IPublishedValueFallback publishedValueFallback, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.PublishedElementExtensions.Value[T](IPublishedElement content, IPublishedValueFallback publishedValueFallback, String alias, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.FriendlyPublishedElementExtensions.Value[T](IPublishedElement content, String alias, String culture, String segment, Fallback fallback, T defaultValue) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_DocTypeGridEditor_T26FullpageInfographicModule.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\DocTypeGridEditor\T26FullpageInfographicModule.cshtml:line 4 at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.ViewViewComponentResult.ExecuteAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentInvoker.InvokeAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentHelper.InvokeCoreAsync(ViewComponentDescriptor descriptor, Object arguments) --- End of inner exception stack trace --- at System.Threading.Tasks.Task.ThrowIfExceptional(Boolean includeTaskCanceledExceptions) at System.Threading.Tasks.Task`1.GetResultCore(Boolean waitCompletionNotification) at Our.Umbraco.DocTypeGridEditor.Helpers.DocTypeGridEditorHelper.RenderDocTypeGridEditorItem(IViewComponentHelper helper, IHtmlHelper htmlHelper, Object model) at AspNetCoreGeneratedDocument.App_Plugins_DocTypeGridEditor_Render_DocTypeGridEditor.ExecuteAsync() at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.RenderPartialCoreAsync(String partialViewName, Object model, ViewDataDictionary viewData, TextWriter writer) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.PartialAsync(String partialViewName, Object model, ViewDataDictionary viewData) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_base.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\base.cshtml:line 5
System.AggregateException: One or more errors occurred. (Value cannot be null. (Parameter 'input')) ---> System.ArgumentNullException: Value cannot be null. (Parameter 'input') at System.Text.RegularExpressions.ThrowHelper.ThrowArgumentNullException(ExceptionArgument arg) at System.Text.RegularExpressions.Regex.Matches(String input) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.FindLocalLinkIds(String text)+MoveNext() at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.Convert(Object source, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) at QBEEurope.Core.ValueConverters.GlobalVariablesValueConverter`1.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Core\ValueConverters\GlobalVariablesValueConverter.cs:line 99 at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.<.ctor>b__7_1() at System.Lazy`1.ViaFactory(LazyThreadSafetyMode mode) at System.Lazy`1.ExecutionAndPublication(LazyHelper executionAndPublication, Boolean useDefaultConstructor) at System.Lazy`1.CreateValue() at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.GetValue(String culture, String segment) at Umbraco.Extensions.PublishedPropertyExtension.Value[T](IPublishedProperty property, IPublishedValueFallback publishedValueFallback, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.PublishedElementExtensions.Value[T](IPublishedElement content, IPublishedValueFallback publishedValueFallback, String alias, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.FriendlyPublishedElementExtensions.Value[T](IPublishedElement content, String alias, String culture, String segment, Fallback fallback, T defaultValue) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_DocTypeGridEditor_T26FullpageInfographicModule.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\DocTypeGridEditor\T26FullpageInfographicModule.cshtml:line 4 at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.ViewViewComponentResult.ExecuteAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentInvoker.InvokeAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentHelper.InvokeCoreAsync(ViewComponentDescriptor descriptor, Object arguments) --- End of inner exception stack trace --- at System.Threading.Tasks.Task.ThrowIfExceptional(Boolean includeTaskCanceledExceptions) at System.Threading.Tasks.Task`1.GetResultCore(Boolean waitCompletionNotification) at Our.Umbraco.DocTypeGridEditor.Helpers.DocTypeGridEditorHelper.RenderDocTypeGridEditorItem(IViewComponentHelper helper, IHtmlHelper htmlHelper, Object model) at AspNetCoreGeneratedDocument.App_Plugins_DocTypeGridEditor_Render_DocTypeGridEditor.ExecuteAsync() at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.RenderPartialCoreAsync(String partialViewName, Object model, ViewDataDictionary viewData, TextWriter writer) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.PartialAsync(String partialViewName, Object model, ViewDataDictionary viewData) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_base.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\base.cshtml:line 5
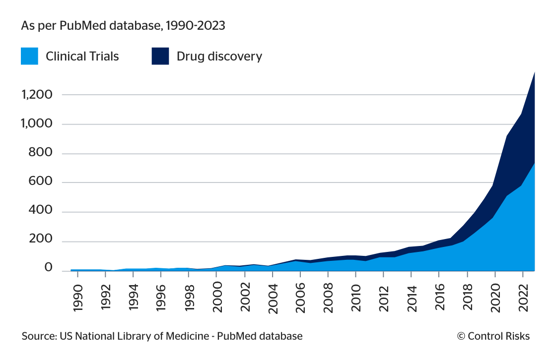
Fig.2: AI mentions in clinical trials, drug discovery studies
Clinical trials
AI also holds the potential to revolutionize clinical trials, which remain marked by challenges and bottlenecks such as recruitment delays, inefficient protocols, and high costs. According to a March 2024 article published in the magazine Nature, over the past six decades the number of drugs approved in the US per billion dollars spent in research and development has halved every nine years. Similar scenarios have been seen in other markets as well, such as Europe, where a combination of red tape and operational complexities has sustained elevated costs for clinical trials and drug development more broadly.
By leveraging AI-driven analytics, researchers can enhance patient recruitment strategies, identify suitable candidates more efficiently, and optimize trial protocols to ensure faster, more reliable results. AI-based predictive modelling will also help researchers to better anticipate patient dropout rates, optimize trial endpoints, and identify biomarkers of treatment response, facilitating more informed decision making throughout the trial process. Finally, AI-driven virtual trials, leveraging real-world data and digital endpoints, have the potential to streamline trial logistics, reduce costs, and enhance participant engagement.
System.AggregateException: One or more errors occurred. (Value cannot be null. (Parameter 'input')) ---> System.ArgumentNullException: Value cannot be null. (Parameter 'input') at System.Text.RegularExpressions.ThrowHelper.ThrowArgumentNullException(ExceptionArgument arg) at System.Text.RegularExpressions.Regex.Matches(String input) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.FindLocalLinkIds(String text)+MoveNext() at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.Convert(Object source, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) at QBEEurope.Core.ValueConverters.GlobalVariablesValueConverter`1.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Core\ValueConverters\GlobalVariablesValueConverter.cs:line 99 at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.<.ctor>b__7_1() at System.Lazy`1.ViaFactory(LazyThreadSafetyMode mode) at System.Lazy`1.ExecutionAndPublication(LazyHelper executionAndPublication, Boolean useDefaultConstructor) at System.Lazy`1.CreateValue() at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.GetValue(String culture, String segment) at Umbraco.Extensions.PublishedPropertyExtension.Value[T](IPublishedProperty property, IPublishedValueFallback publishedValueFallback, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.PublishedElementExtensions.Value[T](IPublishedElement content, IPublishedValueFallback publishedValueFallback, String alias, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.FriendlyPublishedElementExtensions.Value[T](IPublishedElement content, String alias, String culture, String segment, Fallback fallback, T defaultValue) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_DocTypeGridEditor_T26FullpageInfographicModule.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\DocTypeGridEditor\T26FullpageInfographicModule.cshtml:line 4 at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.ViewViewComponentResult.ExecuteAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentInvoker.InvokeAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentHelper.InvokeCoreAsync(ViewComponentDescriptor descriptor, Object arguments) --- End of inner exception stack trace --- at System.Threading.Tasks.Task.ThrowIfExceptional(Boolean includeTaskCanceledExceptions) at System.Threading.Tasks.Task`1.GetResultCore(Boolean waitCompletionNotification) at Our.Umbraco.DocTypeGridEditor.Helpers.DocTypeGridEditorHelper.RenderDocTypeGridEditorItem(IViewComponentHelper helper, IHtmlHelper htmlHelper, Object model) at AspNetCoreGeneratedDocument.App_Plugins_DocTypeGridEditor_Render_DocTypeGridEditor.ExecuteAsync() at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.RenderPartialCoreAsync(String partialViewName, Object model, ViewDataDictionary viewData, TextWriter writer) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.PartialAsync(String partialViewName, Object model, ViewDataDictionary viewData) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_base.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\base.cshtml:line 5
System.AggregateException: One or more errors occurred. (Value cannot be null. (Parameter 'input')) ---> System.ArgumentNullException: Value cannot be null. (Parameter 'input') at System.Text.RegularExpressions.ThrowHelper.ThrowArgumentNullException(ExceptionArgument arg) at System.Text.RegularExpressions.Regex.Matches(String input) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.FindLocalLinkIds(String text)+MoveNext() at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text) at Umbraco.Cms.Core.Templates.HtmlLocalLinkParser.EnsureInternalLinks(String text, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.Convert(Object source, Boolean preview) at Umbraco.Cms.Core.PropertyEditors.ValueConverters.RteMacroRenderingValueConverter.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) at QBEEurope.Core.ValueConverters.GlobalVariablesValueConverter`1.ConvertIntermediateToObject(IPublishedElement owner, IPublishedPropertyType propertyType, PropertyCacheLevel referenceCacheLevel, Object inter, Boolean preview) in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Core\ValueConverters\GlobalVariablesValueConverter.cs:line 99 at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.<.ctor>b__7_1() at System.Lazy`1.ViaFactory(LazyThreadSafetyMode mode) at System.Lazy`1.ExecutionAndPublication(LazyHelper executionAndPublication, Boolean useDefaultConstructor) at System.Lazy`1.CreateValue() at Our.Umbraco.DocTypeGridEditor.Models.DetachedPublishedProperty.GetValue(String culture, String segment) at Umbraco.Extensions.PublishedPropertyExtension.Value[T](IPublishedProperty property, IPublishedValueFallback publishedValueFallback, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.PublishedElementExtensions.Value[T](IPublishedElement content, IPublishedValueFallback publishedValueFallback, String alias, String culture, String segment, Fallback fallback, T defaultValue) at Umbraco.Extensions.FriendlyPublishedElementExtensions.Value[T](IPublishedElement content, String alias, String culture, String segment, Fallback fallback, T defaultValue) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_DocTypeGridEditor_T26FullpageInfographicModule.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\DocTypeGridEditor\T26FullpageInfographicModule.cshtml:line 4 at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.ViewViewComponentResult.ExecuteAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentInvoker.InvokeAsync(ViewComponentContext context) at Microsoft.AspNetCore.Mvc.ViewComponents.DefaultViewComponentHelper.InvokeCoreAsync(ViewComponentDescriptor descriptor, Object arguments) --- End of inner exception stack trace --- at System.Threading.Tasks.Task.ThrowIfExceptional(Boolean includeTaskCanceledExceptions) at System.Threading.Tasks.Task`1.GetResultCore(Boolean waitCompletionNotification) at Our.Umbraco.DocTypeGridEditor.Helpers.DocTypeGridEditorHelper.RenderDocTypeGridEditorItem(IViewComponentHelper helper, IHtmlHelper htmlHelper, Object model) at AspNetCoreGeneratedDocument.App_Plugins_DocTypeGridEditor_Render_DocTypeGridEditor.ExecuteAsync() at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageCoreAsync(IRazorPage page, ViewContext context) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderPageAsync(IRazorPage page, ViewContext context, Boolean invokeViewStarts) at Microsoft.AspNetCore.Mvc.Razor.RazorView.RenderAsync(ViewContext context) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.RenderPartialCoreAsync(String partialViewName, Object model, ViewDataDictionary viewData, TextWriter writer) at Microsoft.AspNetCore.Mvc.ViewFeatures.HtmlHelper.PartialAsync(String partialViewName, Object model, ViewDataDictionary viewData) at AspNetCoreGeneratedDocument.Views_Partials_grid_editors_base.ExecuteAsync() in C:\a\1\s\PublicWebsiteNew.UI\QBEEurope.Web\Views\Partials\grid\editors\base.cshtml:line 5
Fig.2: AI mentions in clinical trials, drug discovery studies

Personalized medicine
The era of one-size-fits-all medicine is giving way, if gradually, to personalized approaches tailored to patients’ unique genetic makeup, lifestyle factors and disease characteristics. AI will enable the analysis of vast genomic datasets and the identification of biomarkers associated with disease susceptibility, prognosis, and treatment response. By leveraging AI-driven precision medicine tools, healthcare providers will be able to optimize treatment strategies, minimize adverse effects, and improve patient outcomes across various medical specialties, from oncology to cardiovascular medicine.
As wearable sensors, mobile health apps and digital biomarkers become increasingly common (particularly in developed economies, as we’re seeing in Europe), real-time data will empower patients in managing health issues before they become critical. By monitoring physiological parameters and receiving personalized recommendations for diet, exercise and stress management, individuals – with the oversight of their doctors – can detect early warning signs of disease and take steps to optimize their wellbeing.
The combination of increased AI sophistication and the continuous expansion of genomic datasets will potentially improve diagnostics, treatment selection, and preventive care.
Medical device customization
Medical devices – ranging from implants to wearable sensors – are undergoing a transformation fuelled by AI-driven customization. By integrating AI algorithms with advanced manufacturing techniques such as 3D printing, medical device manufacturers can create bespoke solutions tailored to individual patient anatomies and needs.
This personalized approach not only enhances device performance and compatibility but minimizes the likelihood of complications and improves patient satisfaction – ultimately impacting the insurance landscape and supporting the process of devices becoming safer and more reliable.
Personalized medicine
The era of one-size-fits-all medicine is giving way, if gradually, to personalized approaches tailored to patients’ unique genetic makeup, lifestyle factors and disease characteristics. AI will enable the analysis of vast genomic datasets and the identification of biomarkers associated with disease susceptibility, prognosis, and treatment response. By leveraging AI-driven precision medicine tools, healthcare providers will be able to optimize treatment strategies, minimize adverse effects, and improve patient outcomes across various medical specialties, from oncology to cardiovascular medicine.
As wearable sensors, mobile health apps and digital biomarkers become increasingly common (particularly in developed economies, as we’re seeing in Europe), real-time data will empower patients in managing health issues before they become critical. By monitoring physiological parameters and receiving personalized recommendations for diet, exercise and stress management, individuals – with the oversight of their doctors – can detect early warning signs of disease and take steps to optimize their wellbeing.
The combination of increased AI sophistication and the continuous expansion of genomic datasets will potentially improve diagnostics, treatment selection, and preventive care.
Medical device customization
Medical devices – ranging from implants to wearable sensors – are undergoing a transformation fuelled by AI-driven customization. By integrating AI algorithms with advanced manufacturing techniques such as 3D printing, medical device manufacturers can create bespoke solutions tailored to individual patient anatomies and needs.
This personalized approach not only enhances device performance and compatibility but minimizes the likelihood of complications and improves patient satisfaction – ultimately impacting the insurance landscape and supporting the process of devices becoming safer and more reliable.
Fig.3: AI and machine learning-enabled devices approved by the FDA, 2013-22
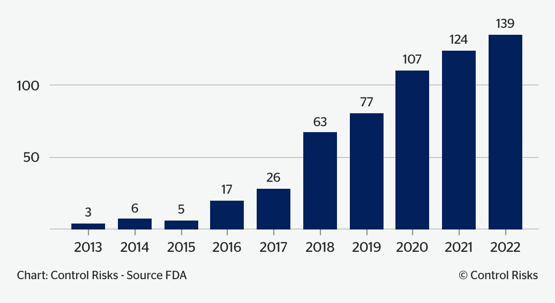
According to its own database, the US Food and Drug Administration (FDA) has approved more than 100 new AI, machine learning-enabled devices per year since 2020 – compared to a few units per year 10 years ago. Over the past year the European Medicines Agency (EMA) has conducted a series of public consultations on the use of AI in the medical product lifecycle, establishing guidelines and initial recommendations for organizations aiming at developing new AI - and machine learning-based medical devices
Regulatory prioritization around AI will likely only intensify in the coming years as more technological disruptions drive both supply and demand in the sector.
One area where AI-driven customization is making strides is orthopaedic implants, including artificial knees and hips. By leveraging AI algorithms to analyze preoperative imaging scans, surgeons can precisely plan the placement and sizing of implants to align with each patient’s unique anatomy. This personalized approach improves the fit and stability of implants while reducing postoperative complications, such as implant loosening or malalignment, and improving wearer’s comfort.
Moreover, AI-driven customization enables the design of implants with complex geometries, enhancing integration and implant durability.
In the UK, researchers associated with the National Health Service (NHS) have published a number of studies in the past two years analyzing AI potentialities in a range of segments, including orthopaedics. In February 2023, the NHS published an official guidance document for patients and healthcare workers on the use and risks of AI, recognizing such innovations as potential drivers of significant advancements to healthcare.
Fig.3: AI and machine learning-enabled devices approved by the FDA, 2013-22

According to its own database, the US Food and Drug Administration (FDA) has approved more than 100 new AI, machine learning-enabled devices per year since 2020 – compared to a few units per year 10 years ago. Over the past year the European Medicines Agency (EMA) has conducted a series of public consultations on the use of AI in the medical product lifecycle, establishing guidelines and initial recommendations for organizations aiming at developing new AI - and machine learning-based medical devices
Regulatory prioritization around AI will likely only intensify in the coming years as more technological disruptions drive both supply and demand in the sector.
One area where AI-driven customization is making strides is orthopaedic implants, including artificial knees and hips. By leveraging AI algorithms to analyze preoperative imaging scans, surgeons can precisely plan the placement and sizing of implants to align with each patient’s unique anatomy. This personalized approach improves the fit and stability of implants while reducing postoperative complications, such as implant loosening or malalignment, and improving wearer’s comfort.
Moreover, AI-driven customization enables the design of implants with complex geometries, enhancing integration and implant durability.
In the UK, researchers associated with the National Health Service (NHS) have published a number of studies in the past two years analyzing AI potentialities in a range of segments, including orthopaedics. In February 2023, the NHS published an official guidance document for patients and healthcare workers on the use and risks of AI, recognizing such innovations as potential drivers of significant advancements to healthcare.
Areas at risk
Unsurprisingly, AI integration into the healthcare industry will have meaningful implications for adjacent industries, including insurance. Policies relevant to the healthcare industry will need to accommodate new stakeholders, and an awareness of the risks associated with AI integration in an environment where there is a huge amount of hype and some unrealistic expectations about the impact of AI on disease eradication and control.
AI integration is bringing new players into the healthcare industry such as technology companies that may have varying degrees of familiarity with risk issues specific to working with patients in healthcare settings. A potential culture clash between the intrinsically cautious approach by healthcare professionals and the risk-taking ethos frequently associated with tech innovation will also add complexity to this evolving relationship. Furthermore, AI integration is also challenging the role and status of established stakeholders in the industry, including regulators that are typically responding slowly and inconsistently across markets to adjust or create rules relevant to governing the use and implications of AI. Evolving approaches to the role of patients themselves, in shaping or directing drug development and administration and the broader provision of healthcare, will drive changes to risk exposure.
Meanwhile, the rapid roll-out of AI across sectors will likely lead to increased malicious activity seeking to manipulate large-language models (LLMs) or access the datasets they rely on in the coming years. These factors alone illustrate the extent to which insurers will need to be nimble as the risk environment relevant to healthcare evolves in the coming years.
The rapid roll-out of AI will likely lead to increased malicious activity seeking to manipulate large language models or access datasets

Unsurprisingly, AI integration into the healthcare industry will have meaningful implications for adjacent industries, including insurance. Policies relevant to the healthcare industry will need to accommodate new stakeholders, and an awareness of the risks associated with AI integration in an environment where there is a huge amount of hype and some unrealistic expectations about the impact of AI on disease eradication and control.
AI integration is bringing new players into the healthcare industry such as technology companies that may have varying degrees of familiarity with risk issues specific to working with patients in healthcare settings. A potential culture clash between the intrinsically cautious approach by healthcare professionals and the risk-taking ethos frequently associated with tech innovation will also add complexity to this evolving relationship. Furthermore, AI integration is also challenging the role and status of established stakeholders in the industry, including regulators that are typically responding slowly and inconsistently across markets to adjust or create rules relevant to governing the use and implications of AI. Evolving approaches to the role of patients themselves, in shaping or directing drug development and administration and the broader provision of healthcare, will drive changes to risk exposure.
Meanwhile, the rapid roll-out of AI across sectors will likely lead to increased malicious activity seeking to manipulate large-language models (LLMs) or access the datasets they rely on in the coming years. These factors alone illustrate the extent to which insurers will need to be nimble as the risk environment relevant to healthcare evolves in the coming years.
The rapid roll-out of AI will likely lead to increased malicious activity seeking to manipulate large language models or access datasets

Risks related to biases are also considerable. AI algorithms can inadvertently perpetuate or even exacerbate existing biases present in the data they are trained on, leading to skewed results that disproportionately affect certain populations. For instance, if the training data lacks diversity, the AI model may underperform or produce inaccurate predictions for underrepresented groups, resulting in healthcare disparities.
Risk management strategies must therefore include rigorous validation of AI tools across diverse datasets, continuous monitoring for biased outcomes, and implementing corrective measures to ensure fair and unbiased applications. Additionally, transparency in AI decision-making processes and involving diverse stakeholders in the development and deployment of these technologies are crucial steps to mitigate ethical risks and promote trust and inclusivity in the life sciences.
Regulatory bodies such as the FDA (USA) and the EMA (Europe) will play a crucial role in establishing guidelines and standards for the development and deployment of AI tools, ensuring they meet stringent safety and efficacy criteria before being approved for use. Independent audits and peer reviews by multidisciplinary teams of experts – including ethicists, data scientists, clinicians, and patient advocates – will be essential to evaluate AI performance and ethical implications. Continuous monitoring and post-market surveillance will help identify and address any issues that arise in real-world applications, ensuring that the benefits of AI in life sciences are maximized while minimizing potential harms.
Risks related to biases are also considerable. AI algorithms can inadvertently perpetuate or even exacerbate existing biases present in the data they are trained on, leading to skewed results that disproportionately affect certain populations. For instance, if the training data lacks diversity, the AI model may underperform or produce inaccurate predictions for underrepresented groups, resulting in healthcare disparities.
Risk management strategies must therefore include rigorous validation of AI tools across diverse datasets, continuous monitoring for biased outcomes, and implementing corrective measures to ensure fair and unbiased applications. Additionally, transparency in AI decision-making processes and involving diverse stakeholders in the development and deployment of these technologies are crucial steps to mitigate ethical risks and promote trust and inclusivity in the life sciences.
Regulatory bodies such as the FDA (USA) and the EMA (Europe) will play a crucial role in establishing guidelines and standards for the development and deployment of AI tools, ensuring they meet stringent safety and efficacy criteria before being approved for use. Independent audits and peer reviews by multidisciplinary teams of experts – including ethicists, data scientists, clinicians, and patient advocates – will be essential to evaluate AI performance and ethical implications. Continuous monitoring and post-market surveillance will help identify and address any issues that arise in real-world applications, ensuring that the benefits of AI in life sciences are maximized while minimizing potential harms.
Data privacy and security
A key assumption behind the benefits of AI in the life sciences world is around its capacity to collect and process large amounts of data – including particularly sensitive datasets such as personally identifiable information (PII) and personal health information (PHI), which are already in the targeting focus of threat actors seeking to exploit data for fraud or extortion. Increased reliance on AI models within the life science industry is therefore likely to expand the potential impact of data breaches, while making the industry – and those providing AI solutions to the industry – more attractive for malicious actors.
As such, data privacy and data security concerns will be on the top of the agenda for regulators, industry players and consumers – particularly in sectors handling sensitive datasets, such as life sciences. The need to safeguard patient privacy will require a combination of robust data governance frameworks and reliable regulatory oversight – as stressed by an October 2023 guideline by the World Health Organization (WHO). There is limited evidence to suggest that these safeguards are in place in most markets, as different stakeholders adapt to new paradigms and challenges.
Machine learning models trained on sensitive medical data can inadvertently learn and retain private information, leading to concerns about data anonymization and re-identification. As AI systems become more sophisticated and capable of processing intricate datasets, there is an elevated risk of anonymized data being reverse engineered to identify individuals.
Regulatory volatility and increasing civil society scrutiny over innovation means that players with stakes in the AI industry will need to closely monitor trends and to develop robust response mechanisms to navigate a more volatile risk environment.
The elevated complexity of certain devices means that even a single incompatibility has the potential to produce errors
Data privacy and security
A key assumption behind the benefits of AI in the life sciences world is around its capacity to collect and process large amounts of data – including particularly sensitive datasets such as personally identifiable information (PII) and personal health information (PHI), which are already in the targeting focus of threat actors seeking to exploit data for fraud or extortion. Increased reliance on AI models within the life science industry is therefore likely to expand the potential impact of data breaches, while making the industry – and those providing AI solutions to the industry – more attractive for malicious actors.
As such, data privacy and data security concerns will be on the top of the agenda for regulators, industry players and consumers – particularly in sectors handling sensitive datasets, such as life sciences. The need to safeguard patient privacy will require a combination of robust data governance frameworks and reliable regulatory oversight – as stressed by an October 2023 guideline by the World Health Organization (WHO). There is limited evidence to suggest that these safeguards are in place in most markets, as different stakeholders adapt to new paradigms and challenges.
Machine learning models trained on sensitive medical data can inadvertently learn and retain private information, leading to concerns about data anonymization and re-identification. As AI systems become more sophisticated and capable of processing intricate datasets, there is an elevated risk of anonymized data being reverse engineered to identify individuals.
Regulatory volatility and increasing civil society scrutiny over innovation means that players with stakes in the AI industry will need to closely monitor trends and to develop robust response mechanisms to navigate a more volatile risk environment.

The elevated complexity of certain devices means that even a single incompatibility has the potential to produce errors
Medical device failure
Although AI will likely produce increasingly accurate outcomes when compared with human-based approaches overall, failures remain possible. For example, a benign tumour might be wrongly identified as malignant (and vice versa) due to glitches in computer vision software. Similarly, the misidentification of an object by software in the context of a surgery might lead to critical mistakes.
Errors might also come from the hardware side of the equation. As companies enter an increasingly competitive space, there will inevitably be a fight for price – and ultimately cost. Sourcing strategies for their materials might favour scale over robustness, which may, in turn, represent a critical reliability risk for end consumers. The elevated complexity of certain devices, which often comprise hundreds of different pieces, means that even a single incompatibility has the potential to produce errors that are hard to mitigate.
Finally, issues related to maintenance – common to devices overall – will remain present in the AI ecosystem, retaining potential to drive risks to patients, practitioners, and insurers.
As a result, potential medical device failures will be in the regulatory spotlight. This has been illustrated by specific provisions targeting the sector in the EU AI Act, which was approved by the European Parliament in March 2024 and will likely set the agenda for similar initiatives elsewhere in the coming months and years. AI systems applied to medical devices are classified as high risk and will continue to receive proportional scrutiny before and after they are put on the market. However, the innovative nature of some systems mean that some failures might still be hard to anticipate, raising meaningful risks for market players.
Training gaps
AI tools are expected to replace the role played by humans in many tasks, but scientists, pharmacologists and healthcare professionals will remain in demand and the skills required will evolve. AI integration into healthcare will require upskilling existing teams and the incorporation of new or different skills into drug development and administration, and the provision of healthcare.
In the near term in particular, training needs may be challenging to meet in the context of pressures on healthcare budgets. In addition, an “AI-rush” – increasing anxiety by private and public organizations to integrate AI tools into their strategies and operations – might compound recruitment and training efforts by distracting resources and leadership – and by potentially compromising existing protocols, including around training and safety.
Human risk
It is worth emphasising that AI integration will not eliminate risks associated with human errors. These will persist in the life sciences sector despite increasing AI adoption – and might even be amplified by new tools in some cases. Human errors in AI contexts might occur due to several reasons, including the unproper understanding of patterns and applications as well as the potential overreliance on complex software in the context of critical decision making.
The fact that some AI systems are based on millions or even billions of lines of codes means that, sometimes, humans might even struggle to identify the origin of potential inconsistencies. This will add uncertainty to patient care and, ultimately, audit and insurance risk management.
Medical device failure
Although AI will likely produce increasingly accurate outcomes when compared with human-based approaches overall, failures remain possible. For example, a benign tumour might be wrongly identified as malignant (and vice versa) due to glitches in computer vision software. Similarly, the misidentification of an object by software in the context of a surgery might lead to critical mistakes.
Errors might also come from the hardware side of the equation. As companies enter an increasingly competitive space, there will inevitably be a fight for price – and ultimately cost. Sourcing strategies for their materials might favour scale over robustness, which may, in turn, represent a critical reliability risk for end consumers. The elevated complexity of certain devices, which often comprise hundreds of different pieces, means that even a single incompatibility has the potential to produce errors that are hard to mitigate.
Finally, issues related to maintenance – common to devices overall – will remain present in the AI ecosystem, retaining potential to drive risks to patients, practitioners, and insurers.
As a result, potential medical device failures will be in the regulatory spotlight. This has been illustrated by specific provisions targeting the sector in the EU AI Act, which was approved by the European Parliament in March 2024 and will likely set the agenda for similar initiatives elsewhere in the coming months and years. AI systems applied to medical devices are classified as high risk and will continue to receive proportional scrutiny before and after they are put on the market. However, the innovative nature of some systems mean that some failures might still be hard to anticipate, raising meaningful risks for market players.
Training gaps
AI tools are expected to replace the role played by humans in many tasks, but scientists, pharmacologists and healthcare professionals will remain in demand and the skills required will evolve. AI integration into healthcare will require upskilling existing teams and the incorporation of new or different skills into drug development and administration, and the provision of healthcare.
In the near term in particular, training needs may be challenging to meet in the context of pressures on healthcare budgets. In addition, an “AI-rush” – increasing anxiety by private and public organizations to integrate AI tools into their strategies and operations – might compound recruitment and training efforts by distracting resources and leadership – and by potentially compromising existing protocols, including around training and safety.
Human risk
It is worth emphasising that AI integration will not eliminate risks associated with human errors. These will persist in the life sciences sector despite increasing AI adoption – and might even be amplified by new tools in some cases. Human errors in AI contexts might occur due to several reasons, including the unproper understanding of patterns and applications as well as the potential overreliance on complex software in the context of critical decision making.
The fact that some AI systems are based on millions or even billions of lines of codes means that, sometimes, humans might even struggle to identify the origin of potential inconsistencies. This will add uncertainty to patient care and, ultimately, audit and insurance risk management.
Outlook
AI is set to promote significant disruptions across the business environment and the day-to-day life in the coming years. From business models to regulatory frameworks and routine tasks, its integration seems inevitable and far-reaching. Navigating such uncharted waters will be a challenge, requiring companies and professionals in different industries and roles to embrace well-informed views regarding innovation, without losing sight of ethical, safety and regulatory considerations.
Although it is hard to forecast the magnitude and range of specific threats that will emerge against this backdrop, the impactful nature of AI-related developments suggests that they deserve proper, proactive attention, and that they will likely become a key aspect of risk management across the economy, including in the life sciences world and particularly around patient centricity.
After all, transformative change has already started, and reported advancements are likely to be just the beginning of a comprehensive, long-lasting trend.
Annex – key references
How AI is being used to accelerate clinical trials, nature.com
How Artificial Intelligence is Revolutionizing Drug Discovery, harvard.edu
AI in healthcare: how could liability arise?, lawscot.org.uk
Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices, fda.gov
The Promise and Potential Pitfalls of AI in Medical Device Design, medtechintelligence.com
What are the legal implications of European AI regulations for medical device companies?, allenovery.com
WHO outlines considerations for regulation of artificial intelligence for health, who.int
Artificial Intelligence Optimizes Recovery and Mobility for Hip Replacement Patients, orlandohealth.com
National Library of Medicine – PubMed, pubmed.ncbi.nlm.nih.gov
AI is set to promote significant disruptions across the business environment and the day-to-day life in the coming years. From business models to regulatory frameworks and routine tasks, its integration seems inevitable and far-reaching. Navigating such uncharted waters will be a challenge, requiring companies and professionals in different industries and roles to embrace well-informed views regarding innovation, without losing sight of ethical, safety and regulatory considerations.
Although it is hard to forecast the magnitude and range of specific threats that will emerge against this backdrop, the impactful nature of AI-related developments suggests that they deserve proper, proactive attention, and that they will likely become a key aspect of risk management across the economy, including in the life sciences world and particularly around patient centricity.
After all, transformative change has already started, and reported advancements are likely to be just the beginning of a comprehensive, long-lasting trend.

Annex – key references
How AI is being used to accelerate clinical trials, nature.com
How Artificial Intelligence is Revolutionizing Drug Discovery, harvard.edu
AI in healthcare: how could liability arise?, lawscot.org.uk
Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices, fda.gov
The Promise and Potential Pitfalls of AI in Medical Device Design, medtechintelligence.com
What are the legal implications of European AI regulations for medical device companies?, allenovery.com
WHO outlines considerations for regulation of artificial intelligence for health, who.int
Artificial Intelligence Optimizes Recovery and Mobility for Hip Replacement Patients, orlandohealth.com
National Library of Medicine – PubMed, pubmed.ncbi.nlm.nih.gov
This report has been developed for QBE by Control Risks
This report has been developed for QBE by Control Risks
Building Supply Chain Resilience
Understand and manage risk in your supply chain
Sign-up to be notified about future articles from the Sector Resilience Series, and other thoughts, reports or insights from QBE.
